Books of Interest
Website: chetyarbrough.blog
“The Nature of Matter: Understanding the Physical World” (The Great Book Lectures)
By: David Ball
Narrated by: Professor David W. Ball

Professor David W. Ball (Professor of Chemistry and Chair of Chemistry Dept. at Cleveland State University, received Masters and Doctoral Degrees from Rice University,
Professor Bell offers a definition and description of matter in the universe. He carries on much of what is explained by Pollock in “Particle Physics”. Bell explains how physics particles form matter with the addition of energy, Bell reifies and expands Pollock’s history of physics. Though there is significant overlap in their presentations, Bell offers a more detailed understanding of matter with its component particles and the role of energy in what humans hear, feel, smell, and see.
Two facts about matter expanded by Bell are about energy’ component’s and structure’s interactions among and within atoms. Though Pollock alluded to the structure of matter and fully explains energy’s importance at the atomic level, Bell expands explanation of electrons and the way they provide energy within and between atoms.

The structure of revolving electrons generate energy in different orbits around the nucleus of an atom. Initially, those orbits were thought to be like planets revolving around the sun but were found to be located within shells around the nucleus in three different orbits. These shells come in three categories. One is spherically symmetric (called the S orbital), the second is dumbbell-like with two lobes along specific axis’s (called P orbitals), and the third (which are also called P orbitals) follow a preferred direction that is not spherical. These shells are important because their reactivity and bonding play a critical role in the formation of matter.
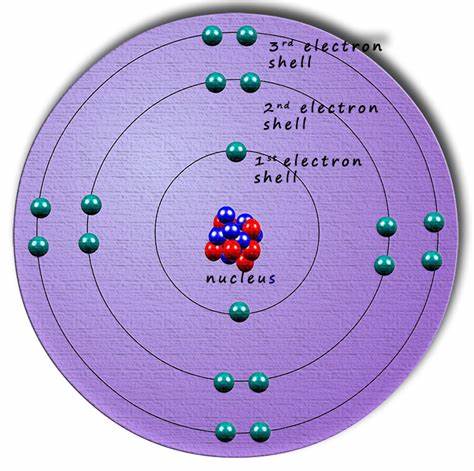
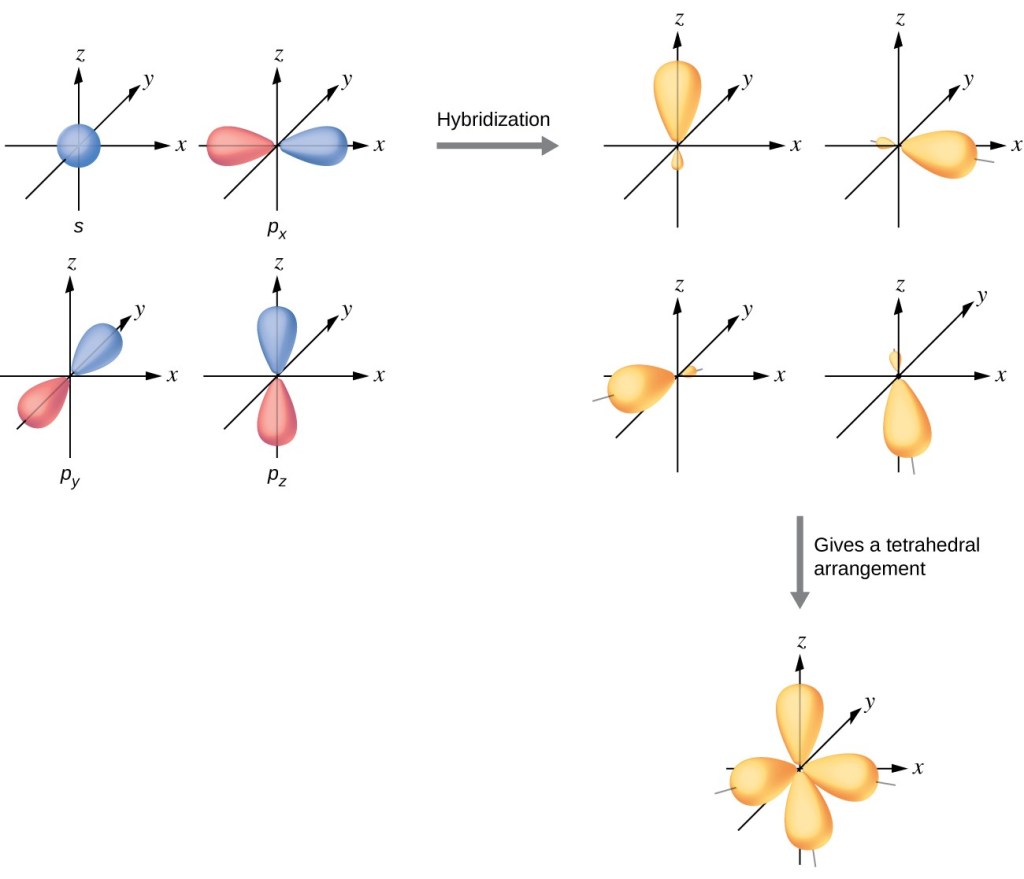
Ball explains electron arrangement around the nucleus of an atom determine chemical properties and behavior of molecular interactions. Electrons are the wave feature of Quantum Mechanics that confound an ordered world of cause and effect postulated by Albert Einstein. What is made a little clearer by Ball is that color is an integral part of energy at the atomic level. Electron energy has discrete and precise energy levels that are arranged around the nucleus of an atom.

Without light particles (protons), energy would not exist. Ball notes electron energy is fundamentally affected by light.

Light or photons are the source of discrete energy levels called quanta that do different things–1) generate absorption, 2) cause transition between shell levels, 3) generate fluorescence, and/or 4) penetrate an atom’s dense nucleus to change mass to energy.
Ball explains why carbon is the most important element in the periodic table. Carbon’s importance is signified by its absence or presence in matter. Matter is either organic or inorganic with carbon being the measure of its classification. The astounding realization is that as a percentage of the earth’s elements, carbon is only 0.032% of our environment. (In contrast, the 3 largest fundamental elements on earth are oxygen at 46.6%, silicon is 27.7%, and Aluminum is 8.1%.) It is a reminder that earth’s living things (organic matter) are dependent on carbon, a miniscule percentage of our environment.

Ball’s chapter on water is an enlightening exploration of its reputation as a universal solvent with various uses and characteristics when boiled or frozen. Water’s dissolving and heat-storing capability are thoughtfully explained. Pollution is touched upon with explanations about what is being done and needs to be improved to preserve the world’s environment.
Ball explores prosthesis and material questions and solutions for the creation of body parts.

From dental fillings to tooth implants, to artificial hips, knees, hearts, arteries and breast implants, Ball explains how biochemistry and materials are critical to their manufacture and utility. He suggests the future will include brain implant enhancements and increases in human longevity.

In “Resistance is Futile”, Ball explains the value of superconductivity.
The current reality of world’ electrification is that 30% of its beneficial power is lost in transmission. Material qualities of our wired world inhibit electrical power conductivity. That 30% loss can be reduced by hugely lowering the temperature of transmission material, with the idea to invent a superconductive material that does not require super-cooled temperatures. Success in finding that material remains a work in progress. No one has found a superconductivity material that does not require super-cooled temperatures. However, Ball notes discovery would be an immense energy saver for the world.
In contrast to “Resistance is Futile” Ball notes “Resistance is Useful”.
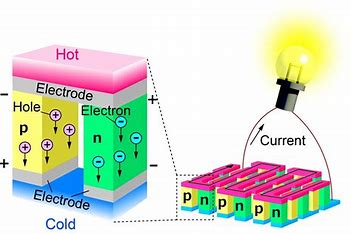
Ball explains how resistance creates heat in a semi-conductor that can be translated in a wired circuit to trigger a directed instrumental behavior or action. With the design of circuit boards with semi-conductors (specifically transistors), one could initiate or complete a series of tasks. From automating machines to creating powerful laptop computers, semi-conductor manufacture grew into an immense industry. As the complexity of tasks increased, the size of semi-conductors decreased. Gordon Moore proposed Moore’s law that suggested transistor’ size (a form of semi-conductor) in integrated circuits would become smaller and double every two years. Moore’s Law is not precisely true, but miniaturization, performance, and integration remain semi-conductor manufacturing’ goals.

The last lectures address composites and their component assembly in everything from concrete to fiberglass to tires.
These composites are formed from different materials based on their elemental properties that provide valuable materials to society. They are formed by atomic level interactions between elemental properties. Composite materials are noted as a boon and bane of society. The boon is their utility for new products for work and play. Their bane is disposal and their effect on the environment.

